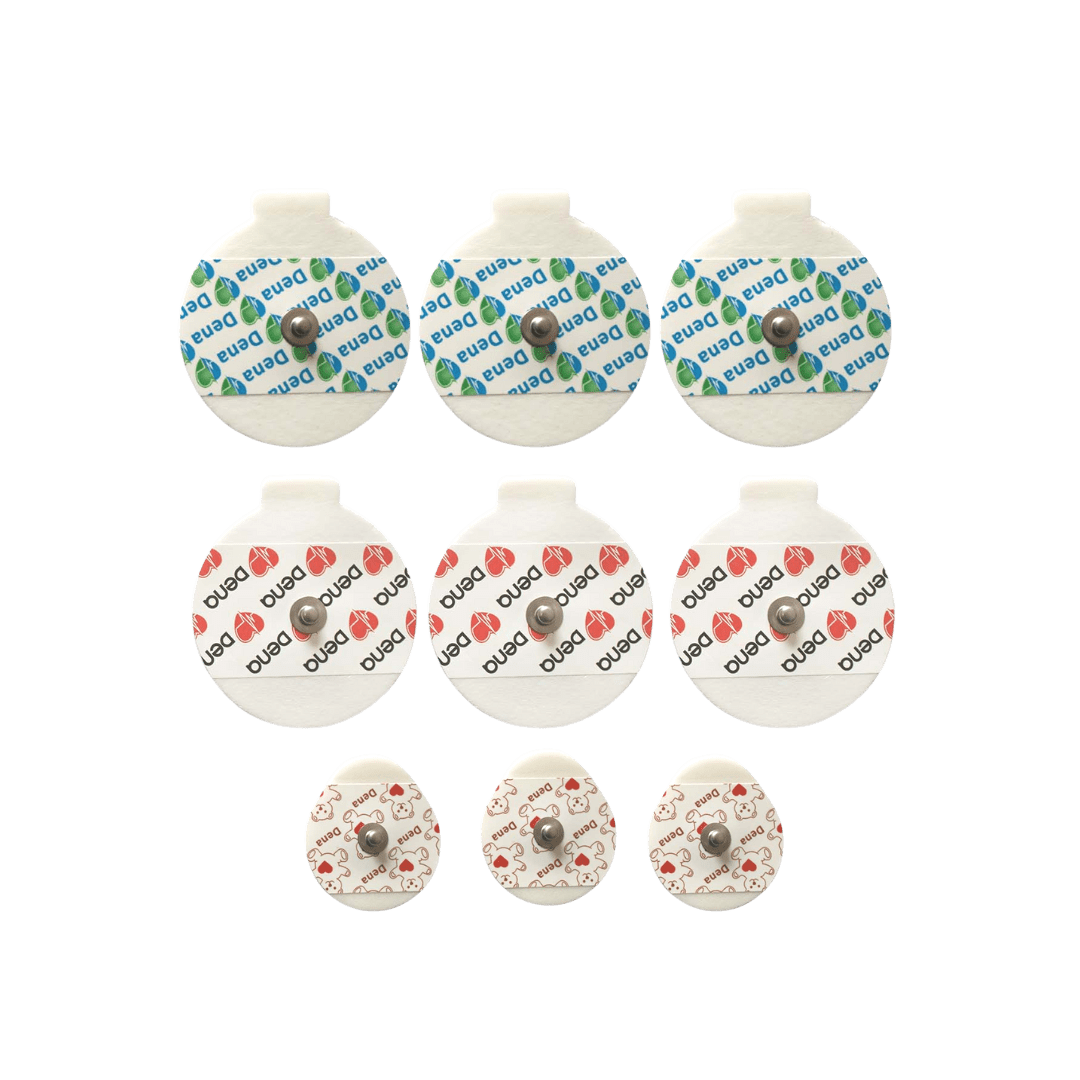
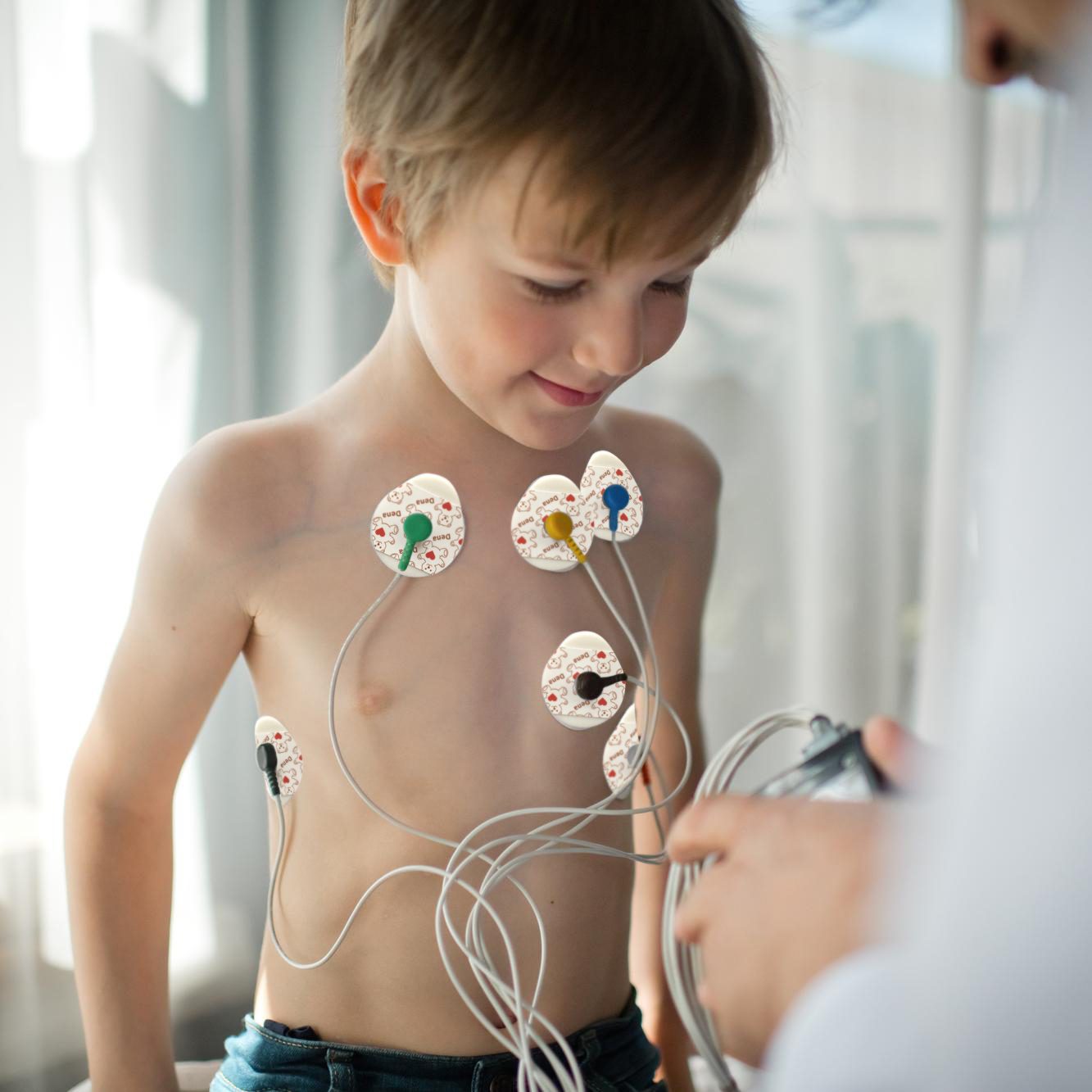
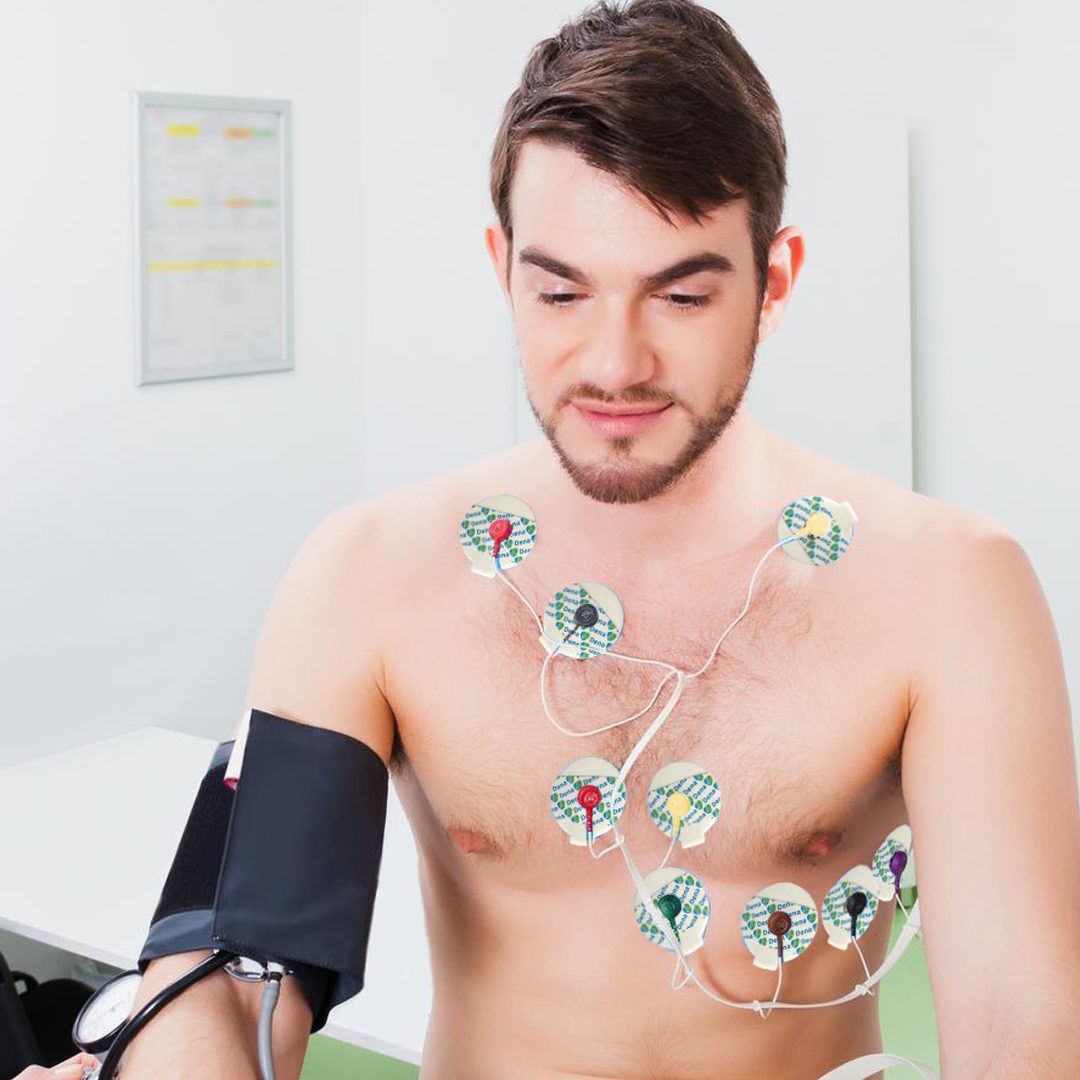
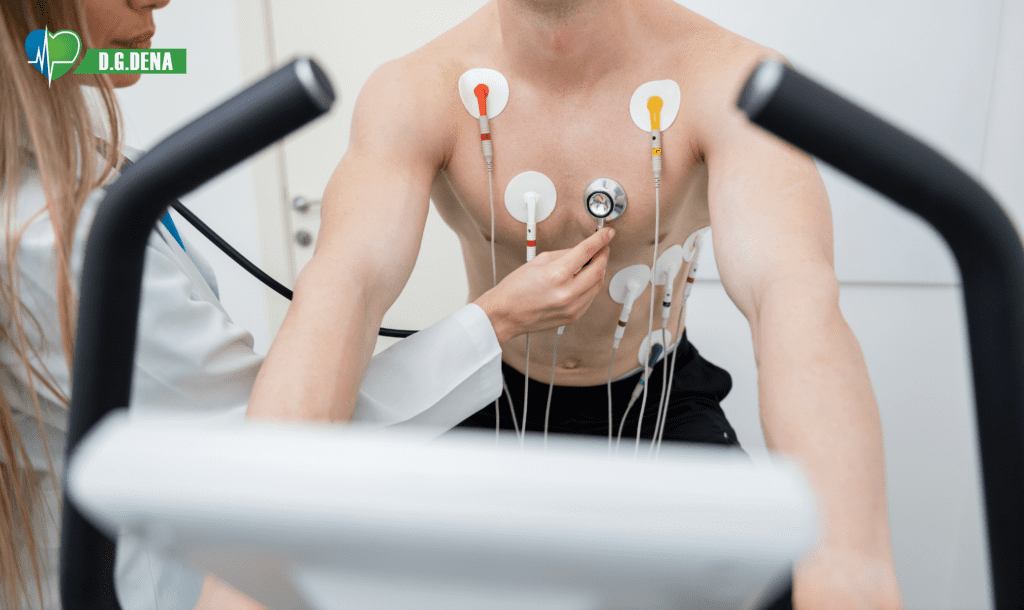
ECG Electrode is a type of conductive electrode in a pad form. The purpose of ECG Electrode is to transmit heart signals by connecting the wires of a cardiac monitoring device to the chest wall. These single-use ECG Electrodes are made of foam. They transmit heart rhythm and pulse to the cardiac monitoring device without any noise or interference. These ECG Electrodes are adhesive and retain their adhesive properties. They are completely hypoallergenic and allergy-free. ECG Electrodes are used in cardiac stress test, cardiac rehabilitation, monitoring, electrocardiography, and Holter monitoring. D.G. Dena ECG Electrodes are available in three different sizes with solid or liquid gel.
Direction for use
Wash your hands and ask the patient to shave the areas you want to apply the ECG Electrode since body hair can interfere with transmitting heart signals. Then remove the transparent plastic cover of the product and apply it on the considered area. Now, you can connect the monitoring device wires to the ECG Electrode Studs.